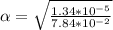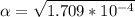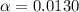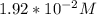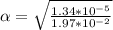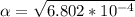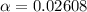Chemistry, 25.02.2020 22:51 tmmackie1748
Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following concentrations (Ka is given in Appendix D in the textbook).
Part A. 0.270 M
Part B. 7.84×10-2 M
Part C. 1.97×10-2 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following...
Questions

Mathematics, 23.08.2021 09:10


English, 23.08.2021 09:10



English, 23.08.2021 09:10


Mathematics, 23.08.2021 09:10


Mathematics, 23.08.2021 09:10



History, 23.08.2021 09:10

Biology, 23.08.2021 09:10


Physics, 23.08.2021 09:10

Mathematics, 23.08.2021 09:10


Biology, 23.08.2021 09:10

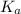 =
= 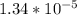
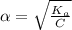
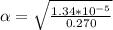
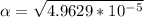
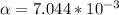
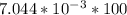
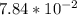 M
M