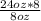Chemistry, 25.02.2020 23:17 shelbycg02
Your pharmacy stocks solution 'Q' in an 8oz concentrated bottle. Solution 'Q' is usually ordered in 8%. Consequently, your stock bottles are diluted with 16oz of distilled water to prepare 24oz of 8% solution 'Q' in a 1L glass beaker. What is the concentration of the stock bottle?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Your pharmacy stocks solution 'Q' in an 8oz concentrated bottle. Solution 'Q' is usually ordered in...
Questions

History, 22.10.2020 06:01


History, 22.10.2020 06:01


Mathematics, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01

History, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01

Arts, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01


English, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01



Mathematics, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01


History, 22.10.2020 06:01