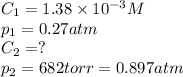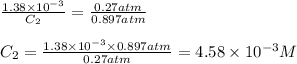Chemistry, 26.02.2020 00:21 amadileaks
The solubility of o2 at 20c is 1.38 x10^-3. the partial presure of o2 in the air at sea level is 0.27 atm. using henery;s law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20C and an atmospheric pressure of 682 TM

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
The solubility of o2 at 20c is 1.38 x10^-3. the partial presure of o2 in the air at sea level is 0.2...
Questions




Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00



Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


Social Studies, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


History, 13.05.2021 01:00

Mathematics, 13.05.2021 01:00


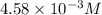
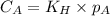
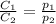
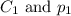 are the initial concentration and partial pressure of oxygen gas
are the initial concentration and partial pressure of oxygen gas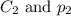 are the final concentration and partial pressure of oxygen gas
are the final concentration and partial pressure of oxygen gas