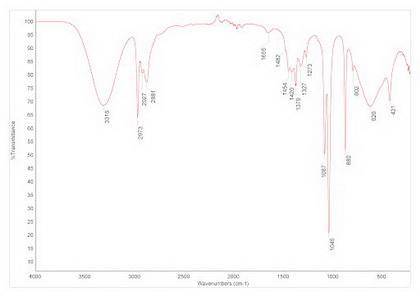
In a fischer esterification, a carboxylic acid and an alcohol combine in the presence of acid to make an ester and a molecule of water. the infrared spectrum shown below represents the substance isolated at the end of the reaction. use the infrared spectrum to identify the substance, and infer the success of the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
In a fischer esterification, a carboxylic acid and an alcohol combine in the presence of acid to mak...
Questions

Mathematics, 25.12.2019 07:31


History, 25.12.2019 07:31


Mathematics, 25.12.2019 07:31

English, 25.12.2019 07:31


Mathematics, 25.12.2019 07:31


History, 25.12.2019 07:31

History, 25.12.2019 07:31


Mathematics, 25.12.2019 07:31

Biology, 25.12.2019 07:31

Mathematics, 25.12.2019 07:31


Mathematics, 25.12.2019 07:31