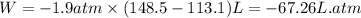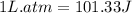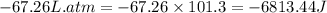Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

You know the right answer?
The combustion of 0.25 moles of octane gas (C8H18) to CO2 gas and H2O gas (vapor) against a constant...
Questions

History, 12.06.2020 05:57


Chemistry, 12.06.2020 05:57

Mathematics, 12.06.2020 05:57


Mathematics, 12.06.2020 05:57

Mathematics, 12.06.2020 05:57

Mathematics, 12.06.2020 05:57


English, 12.06.2020 05:57


Mathematics, 12.06.2020 05:57


Social Studies, 12.06.2020 05:57






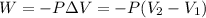
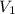 = initial volume = 113.1 L
= initial volume = 113.1 L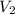 = final volume = 148.5 L
= final volume = 148.5 L