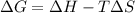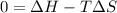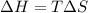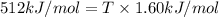As an approximation we can assume that proteins exist either in the native state and the denatured state. The standard molar enthalpy and entropy of the denaturation of a certain protein are 512 kj/mol and 1.60 kj/K mol. comment on the signs and magnitude of these quantities , and calculates the temperature at which the process favors the denatured state.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 09:30
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2
You know the right answer?
As an approximation we can assume that proteins exist either in the native state and the denatured s...
Questions


Arts, 04.12.2020 04:50

English, 04.12.2020 04:50






History, 04.12.2020 04:50

History, 04.12.2020 04:50


Social Studies, 04.12.2020 04:50


Mathematics, 04.12.2020 05:00




Geography, 04.12.2020 05:00