
Chemistry, 26.02.2020 01:51 kerarucker12pe384k
If an equal number of moles of reactants are used, do the following equilibrium mixtures contain primarily reactants or products? Please note this question has 1 submission for each part. (a) HCN(aq) + H2O(l) equilibrium reaction arrow CN −(aq) + H3O+(aq) K = 6.2 10-10 products reactants (b) H2(g) + Cl2(g) equilibrium reaction arrow 2 HCl(g) K = 2.51 104 reactants products

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
If an equal number of moles of reactants are used, do the following equilibrium mixtures contain pri...
Questions

History, 12.10.2019 00:30




Mathematics, 12.10.2019 00:30

Mathematics, 12.10.2019 00:30







Mathematics, 12.10.2019 00:30

Mathematics, 12.10.2019 00:30


Social Studies, 12.10.2019 00:30


English, 12.10.2019 00:30


Mathematics, 12.10.2019 00:30

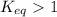 ; the reaction is product favored.When
; the reaction is product favored.When 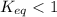 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 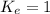 ; the reaction is in equilibrium.
; the reaction is in equilibrium.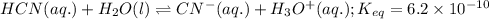
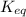 for above reaction follows:
for above reaction follows:![K_{eq}=\frac{[CN^-][H_3O^+]}{[HCN][H_2O]}=6.2\times 10^{-10}](/tpl/images/0524/2318/abbf6.png)
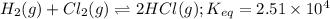
![K_{eq}=\frac{[HCl]^2}{[H_2][Cl_2]}=2.51\times 10^{4}](/tpl/images/0524/2318/4af57.png)


