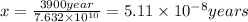Chemistry, 26.02.2020 02:28 ksanquist8896
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the introduction of a catalyst. If the uncatalyzed reaction takes about 3900 years to occur, about how long will the catalyzed reaction take?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
The activation energy for a reaction is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the intr...
Questions


History, 24.06.2019 12:00

English, 24.06.2019 12:00

History, 24.06.2019 12:00

History, 24.06.2019 12:00



History, 24.06.2019 12:00


Mathematics, 24.06.2019 12:00



English, 24.06.2019 12:00


Mathematics, 24.06.2019 12:00





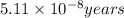 .
.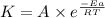
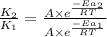
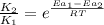
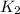 = rate of reaction with catalyst
= rate of reaction with catalyst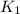 = rate of reaction without catalyst
= rate of reaction without catalyst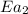 = activation energy with catalyst = 59.0 kJ/mol = 59000 J/mol
= activation energy with catalyst = 59.0 kJ/mol = 59000 J/mol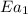 = activation energy without catalyst = 184 kJ/mol = 184000 J/mol
= activation energy without catalyst = 184 kJ/mol = 184000 J/mol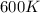
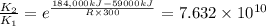
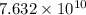 when catalyst is present.
when catalyst is present.