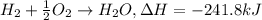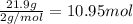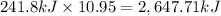Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Acompound is found to contain 6.1% hydrogen and 93.9% oxygen. find it’s empirical formula.
Answers: 2

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
You know the right answer?
When 1 mole of hydrogen gas (H2) reacts with excess oxygen to form water at a constant pressure, 241...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01