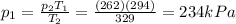On a summer day, you take a road trip through Death Valley, California, in an antique car. You start out at a temperature of 21°C, but the temperature in Death Valley will reach a peak of 56°C. The tires on your car hold 13.2 L of nitrogen gas at a starting pressure of 240 kPa. The tires will burst when the internal pressure (Pb) reaches 262 kPa. Answer the following questions and show your work.
• How many moles of nitrogen gas are in each tire?
• What will the tire pressure be at peak temperature in Death Valley?
• Will the tires burst in Death Valley? Explain.
• If you must let nitrogen gas out of the tire before you go, to what pressure must you reduce the tires before you start your trip? (Assume no significant change in tire volume.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
On a summer day, you take a road trip through Death Valley, California, in an antique car. You start...
Questions


Mathematics, 13.06.2021 07:50

Mathematics, 13.06.2021 07:50





English, 13.06.2021 08:00


Mathematics, 13.06.2021 08:00

French, 13.06.2021 08:00



Mathematics, 13.06.2021 08:00


Mathematics, 13.06.2021 08:00

Biology, 13.06.2021 08:00

Social Studies, 13.06.2021 08:00

Physics, 13.06.2021 08:00

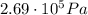
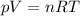
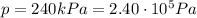
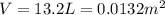
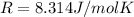
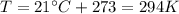
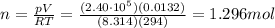
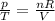
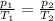
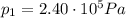 is the initial pressure
is the initial pressure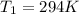 is the initial temperature
is the initial temperature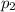 is the final pressure in Death Valley
is the final pressure in Death Valley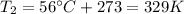 is the temperature in Death Valley
is the temperature in Death Valley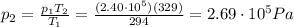
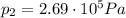
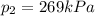
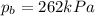
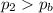
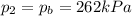
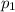 , the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
, the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.