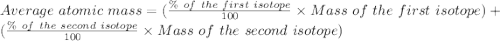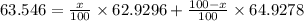Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Copper has two naturally occurring isotopes with atomic masses of 62.9296 u () and 64.9278 u (). The...
Questions

Mathematics, 14.05.2021 23:00



Mathematics, 14.05.2021 23:00


Arts, 14.05.2021 23:00

Mathematics, 14.05.2021 23:00



Geography, 14.05.2021 23:00

Mathematics, 14.05.2021 23:00

English, 14.05.2021 23:00

Mathematics, 14.05.2021 23:00


Mathematics, 14.05.2021 23:00


Mathematics, 14.05.2021 23:00


Social Studies, 14.05.2021 23:00

Mathematics, 14.05.2021 23:00