2A → B + C
The above reaction is run and found to follow second order kinetics with a r...

Chemistry, 26.02.2020 05:54 ranchgirljls
2A → B + C
The above reaction is run and found to follow second order kinetics with a rate constant of 1.30 x 10-3 M-1sec-1. If the initial concentration of A is 1.68 M, what is the concentration after 195 seconds

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Questions

Mathematics, 20.09.2020 18:01

English, 20.09.2020 18:01




Social Studies, 20.09.2020 18:01


Spanish, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01

History, 20.09.2020 18:01

English, 20.09.2020 18:01


Mathematics, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01

Chemistry, 20.09.2020 18:01





History, 20.09.2020 18:01

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0524/6882/ccade.png)
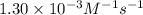
![[A_t]](/tpl/images/0524/6882/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0524/6882/dc622.png) = initial concentration = 1.68 M
= initial concentration = 1.68 M![1.30\times 10^{-3}\times 195=\frac{1}{[A_t]}-\frac{1}{1.68}](/tpl/images/0524/6882/51288.png)
![[A_t]=1.18M](/tpl/images/0524/6882/84e39.png)


