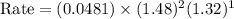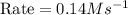For the reaction, A(g) + B(g) => AB(g), the rate is 0.385 mol/L. s when the initial concentrations of both A and B are 2.00 mol/L. If the reaction is second order in A and first order in B, what is the rate when the initial concentration of A = 1.48 mol/L and that of B = 1.32 mol/L. Give your answer to 2 decimal places

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
For the reaction, A(g) + B(g) => AB(g), the rate is 0.385 mol/L. s when the initial concentration...
Questions



Mathematics, 30.11.2020 08:10


Mathematics, 30.11.2020 08:10



Mathematics, 30.11.2020 08:20



Spanish, 30.11.2020 08:20

Chemistry, 30.11.2020 08:20

Mathematics, 30.11.2020 08:30


Mathematics, 30.11.2020 08:30


Mathematics, 30.11.2020 08:30


English, 30.11.2020 08:30

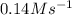
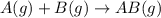
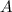 and
and 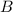 are the reactants.
are the reactants.![\text{Rate}=k[A]^2[B]^1](/tpl/images/0524/7200/858f4.png)
![\text{Rate}=k[A]^2[B]](/tpl/images/0524/7200/15407.png)
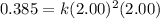
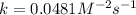
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0524/7200/54afd.png)