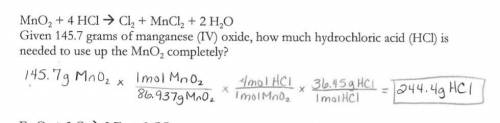MnO2 + 4 HCI + Cl2 + MnCl2 + 2 H20
Given 145.7 grams of manganese (IV) oxide, how much h...

Chemistry, 26.02.2020 13:07 emopandabogard4296
MnO2 + 4 HCI + Cl2 + MnCl2 + 2 H20
Given 145.7 grams of manganese (IV) oxide, how much hydrochloric acid (HCI) is
needed to use up the MnO2 completely?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Questions

Mathematics, 21.09.2020 14:01


Arts, 21.09.2020 14:01


History, 21.09.2020 14:01


Mathematics, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01