Chemistry, 26.02.2020 16:11 lanaasad7292
Nitric oxide is made from the oxidation of ammonia. What mass of nitric oxide can be made from the reaction of 8.00 g NH 3 with 17.0 g O 2? 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Nitric oxide is made from the oxidation of ammonia. What mass of nitric oxide can be made from the r...
Questions

History, 29.01.2020 10:42

Biology, 29.01.2020 10:42




Geography, 29.01.2020 10:42


Physics, 29.01.2020 10:42

Mathematics, 29.01.2020 10:42






Mathematics, 29.01.2020 10:42

Social Studies, 29.01.2020 10:42

History, 29.01.2020 10:42



Mathematics, 29.01.2020 10:43

 .....(1)
.....(1)
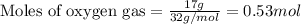

 of ammonia
of ammonia of NO
of NO