
Chemistry, 26.02.2020 17:00 manillasoccer
Consider the following balanced chemical equation. 4 KO 2 + 2 H 2 O ⟶ 4 KOH + 3 O 2 How is the rate of appearance of O 2 , Δ [ O 2 ] Δ t , related to the rate of disappearance of KO 2 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Consider the following balanced chemical equation. 4 KO 2 + 2 H 2 O ⟶ 4 KOH + 3 O 2 How is the rate...
Questions

History, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00


Mathematics, 13.07.2019 01:00


Mathematics, 13.07.2019 01:00



Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00



Chemistry, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

Computers and Technology, 13.07.2019 01:00


Mathematics, 13.07.2019 01:00

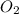 related to the rate of disappearance of
related to the rate of disappearance of 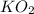 is,
is,![\frac{d[O_2]}{dt}=-\frac{3}{4}\frac{d[KO_2]}{dt}](/tpl/images/0524/9521/55d25.png)
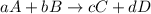
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0524/9521/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0524/9521/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0524/9521/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0524/9521/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0524/9521/d4b94.png)
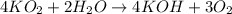
![\text{Rate of disappearance of }KO_2=-\frac{1}{4}\frac{d[KO_2]}{dt}](/tpl/images/0524/9521/4cd78.png)
![\text{Rate of disappearance of }H_2O=-\frac{1}{2}\frac{d[H_2O]}{dt}](/tpl/images/0524/9521/6a91b.png)
![\text{Rate of formation of }KOH=+\frac{1}{4}\frac{d[KOH]}{dt}](/tpl/images/0524/9521/9b206.png)
![\text{Rate of formation of }O_2=+\frac{1}{3}\frac{d[O_2]}{dt}](/tpl/images/0524/9521/e5480.png)
![\text{Rate of reaction}=-\frac{1}{4}\frac{d[KO_2]}{dt}=-\frac{1}{2}\frac{d[H_2O]}{dt}=+\frac{1}{4}\frac{d[KOH]}{dt}=+\frac{1}{3}\frac{d[O_2]}{dt}](/tpl/images/0524/9521/7ae32.png)
![-\frac{1}{4}\frac{d[KO_2]}{dt}=+\frac{1}{3}\frac{d[O_2]}{dt}](/tpl/images/0524/9521/ab2f5.png)
![+\frac{1}{3}\frac{d[O_2]}{dt}=-\frac{1}{4}\frac{d[KO_2]}{dt}](/tpl/images/0524/9521/4e479.png)


