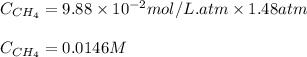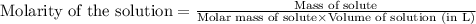Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Methane (CH4, molar mass = 16.0 g/mol) has a Henry's Law constant (kH) of 9.88 × 10–2 mol/(L·atm) wh...
Questions


English, 17.12.2021 01:00

Biology, 17.12.2021 01:00

Mathematics, 17.12.2021 01:00



Mathematics, 17.12.2021 01:00






Mathematics, 17.12.2021 01:00


Mathematics, 17.12.2021 01:00


History, 17.12.2021 01:00



Geography, 17.12.2021 01:00


 = Henry's constant =
= Henry's constant = 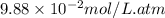
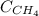 = molar solubility of methane gas = ?
= molar solubility of methane gas = ?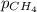 = partial pressure of methane gas = 1.48 atm
= partial pressure of methane gas = 1.48 atm