Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1

Chemistry, 23.06.2019 23:00
Ascientist observes a fault where the hanging wall has moved upward relative to the footwall. which type of fault is the scientist observing? normal reverse strike-slip horizontal
Answers: 1
You know the right answer?
The rate constants of some reactions double with every 10 degree rise in temperature. Assume that a...
Questions

Physics, 15.01.2020 20:31





Chemistry, 15.01.2020 20:31





Business, 15.01.2020 20:31


Mathematics, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31




History, 15.01.2020 20:31


Mathematics, 15.01.2020 20:31

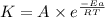
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0524/9978/6d953.png)
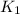 = rate constant at 271 K
= rate constant at 271 K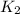 = rate constant at 281 K =
= rate constant at 281 K = 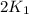
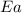 = activation energy for the reaction = ?
= activation energy for the reaction = ?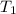 = initial temperature = 271 K
= initial temperature = 271 K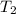 = final temperature = 281 K
= final temperature = 281 K![\log (\frac{2K_1}{K_1})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{271K}-\frac{1}{281K}]](/tpl/images/0524/9978/ceafb.png)