
Chemistry, 26.02.2020 17:47 clairebear66
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the rate of formation of O2 is 7.78 x 10-1 M/s, what is the rate of the loss of O3? 2 O3(g) → 3 O2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3


Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the r...
Questions

Mathematics, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40

English, 03.03.2021 20:40



Computers and Technology, 03.03.2021 20:40

Geography, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40


Mathematics, 03.03.2021 20:40

Mathematics, 03.03.2021 20:40


Advanced Placement (AP), 03.03.2021 20:40


History, 03.03.2021 20:40


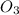 is 0.52M/s
is 0.52M/s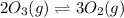
![-\frac{1d[O_3]}{2dt}](/tpl/images/0525/0164/0b459.png)
 =
=![+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/4dcb2.png)
![-\frac{1d[O_3]}{2dt}=+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/b3aa3.png)
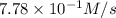
![\frac{2d[O_2]}{3dt}=\frac{2}{3}\times 7.78\times 10^{-1}M/s=0.52M/s](/tpl/images/0525/0164/964d0.png)


