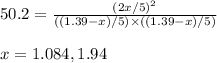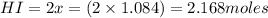Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

You know the right answer?
Calculate the number of moles of HI that are at equilibrium with 1.39 mol of H2 and 1.39 mol of I2 i...
Questions

Mathematics, 03.11.2020 19:30

Mathematics, 03.11.2020 19:30







English, 03.11.2020 19:30



Mathematics, 03.11.2020 19:30


Biology, 03.11.2020 19:30

History, 03.11.2020 19:30

Mathematics, 03.11.2020 19:30

Mathematics, 03.11.2020 19:30

Geography, 03.11.2020 19:30

History, 03.11.2020 19:30

English, 03.11.2020 19:30

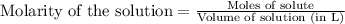
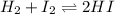
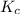 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0525/0428/8acbe.png)
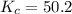
![[HI]_{eq}=\frac{2x}{5.00}](/tpl/images/0525/0428/ab69d.png)
![[H_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/598c6.png)
![[I_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/0f5f3.png)