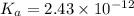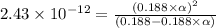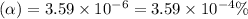Chemistry, 26.02.2020 18:25 blueflu5120
What is the percent ionization of a monoprotic weak acid solution that is 0.188 M? The acid-dissociation (or ionization) constant, Ka, of this acid is 2.43 × 10 − 12 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
What is the percent ionization of a monoprotic weak acid solution that is 0.188 M? The acid-dissocia...
Questions



Biology, 11.08.2021 18:20


Mathematics, 11.08.2021 18:20






Mathematics, 11.08.2021 18:20

Computers and Technology, 11.08.2021 18:20


Social Studies, 11.08.2021 18:20



English, 11.08.2021 18:20

History, 11.08.2021 18:20

Chemistry, 11.08.2021 18:20

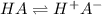
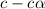
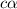
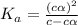
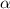 = ?
= ?