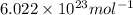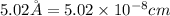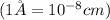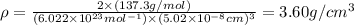Chemistry, 26.02.2020 19:16 hailiemanuel3461
Barium crystallizes in a body-centered cubic system with atoms at all lattice points and an edge length of 5.02 angstroms. Calculate its density in g/cm3.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Barium crystallizes in a body-centered cubic system with atoms at all lattice points and an edge len...
Questions

Mathematics, 18.02.2021 19:00

Chemistry, 18.02.2021 19:00

Mathematics, 18.02.2021 19:00

Mathematics, 18.02.2021 19:00

Mathematics, 18.02.2021 19:00

Mathematics, 18.02.2021 19:00

Chemistry, 18.02.2021 19:00




Arts, 18.02.2021 19:00



Mathematics, 18.02.2021 19:00


Mathematics, 18.02.2021 19:00




Mathematics, 18.02.2021 19:00

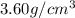
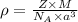 .............(1)
.............(1)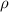 = density = ?
= density = ?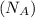 = Avogadro's number =
= Avogadro's number =