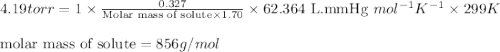A saturated solution is made by dissolving 0.327 g of a polypeptide (a substance formed by joining together in a chainlike fashion some number of amino acids) in water to give 1.70 L of solution. The solution has an osmotic pressure of 4.19 torr at 26 °C. What is the approximate molecular mass of the polypeptide?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
A saturated solution is made by dissolving 0.327 g of a polypeptide (a substance formed by joining t...
Questions

History, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00

History, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00

Computers and Technology, 26.06.2019 07:00

Social Studies, 26.06.2019 07:00

English, 26.06.2019 07:00



History, 26.06.2019 07:00





Mathematics, 26.06.2019 07:00




Geography, 26.06.2019 07:00

Mathematics, 26.06.2019 07:00

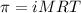
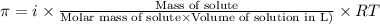
 = osmotic pressure of the solution = 4.19 torr
= osmotic pressure of the solution = 4.19 torr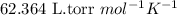
![26^oC=[273+26]K=299K](/tpl/images/0525/2386/ee574.png)