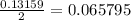Chemistry, 26.02.2020 19:29 whitneyt3218
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants. Part A 5.0 g Ti, 5.0 g F2 Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for ea...
Questions


Mathematics, 07.01.2020 13:31


Social Studies, 07.01.2020 13:31


Mathematics, 07.01.2020 13:31

History, 07.01.2020 13:31


Spanish, 07.01.2020 13:31

Geography, 07.01.2020 13:31



History, 07.01.2020 13:31

Biology, 07.01.2020 13:31

Social Studies, 07.01.2020 13:31


Mathematics, 07.01.2020 13:31

English, 07.01.2020 13:31


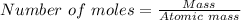
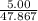
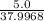
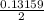 mole of Ti will react with the 0.13159 mole of F₂
mole of Ti will react with the 0.13159 mole of F₂