
Chemistry, 26.02.2020 20:27 peachesmims1991
Consider the reaction 5 Br− (aq) + BrO3− (aq) + 6 H+ (aq) → 3 Br2 (aq) + 3 H2O (l) The average rate of consumption of Br− is 1.81 M/s over the first two minutes. What is the average rate of consumption of H+ during the same time interval?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

You know the right answer?
Consider the reaction 5 Br− (aq) + BrO3− (aq) + 6 H+ (aq) → 3 Br2 (aq) + 3 H2O (l) The average rate...
Questions

Mathematics, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30




Mathematics, 12.07.2019 22:30

Biology, 12.07.2019 22:30

English, 12.07.2019 22:30



Social Studies, 12.07.2019 22:30

Social Studies, 12.07.2019 22:30


Social Studies, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30

Biology, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30

Business, 12.07.2019 22:30


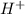 during the same time interval is, 2.17 M/s
during the same time interval is, 2.17 M/s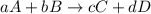
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0525/3531/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0525/3531/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0525/3531/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0525/3531/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0525/3531/d4b94.png)
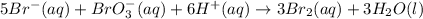
![\text{Rate of disappearance of }Br^-=-\frac{1}{5}\frac{d[Br^-]}{dt}](/tpl/images/0525/3531/86e92.png)
![\text{Rate of disappearance of }BrO_3^-=-\frac{d[BrO_3^-]}{dt}](/tpl/images/0525/3531/4a0f1.png)
![\text{Rate of disappearance of }H^+=-\frac{1}{6}\frac{d[H^+]}{dt}](/tpl/images/0525/3531/09d2b.png)
![\text{Rate of formation of }Br_2=+\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0525/3531/81dff.png)
![\text{Rate of formation of }H_2O=+\frac{1}{3}\frac{d[H_2O]}{dt}](/tpl/images/0525/3531/a608b.png)
![\frac{d[Br^-]}{dt}=1.81M/s](/tpl/images/0525/3531/ab0cf.png)
![-\frac{1}{5}\frac{d[Br^-]}{dt}=-\frac{1}{6}\frac{d[H^+]}{dt}](/tpl/images/0525/3531/b95e1.png)
![-\frac{1}{6}\frac{d[H^+]}{dt}=-\frac{1}{5}\frac{d[Br^-]}{dt}](/tpl/images/0525/3531/980e4.png)
![\frac{d[H^+]}{dt}=\frac{6}{5}\frac{d[Br^-]}{dt}](/tpl/images/0525/3531/ac188.png)
![\frac{d[H^+]}{dt}=\frac{6}{5}\times (1.81M/s)](/tpl/images/0525/3531/11bc3.png)
![\frac{d[H^+]}{dt}=2.17M/s](/tpl/images/0525/3531/1e2e9.png)



