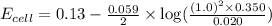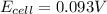Chemistry, 26.02.2020 20:54 adazeb2003
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1.00 M) || Sn2+ (aq, 0.350 M) | Sn4+ (aq, 0.020 M) | Pt (s)The standard reduction potentials are as follows:Sn4+ (aq) + 2 e–à Sn2+ (aq) Eº = +0.13 V2 H+ (aq) + 2 e–à H2 (g) Eº = 0.00 V

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1...
Questions

Mathematics, 08.12.2020 22:00


Mathematics, 08.12.2020 22:00

Physics, 08.12.2020 22:00


World Languages, 08.12.2020 22:00

Mathematics, 08.12.2020 22:00




Mathematics, 08.12.2020 22:00



Mathematics, 08.12.2020 22:00


History, 08.12.2020 22:00

English, 08.12.2020 22:00



Computers and Technology, 08.12.2020 22:00

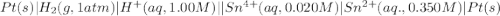
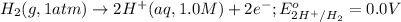
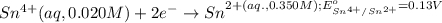
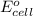 of the reaction, we use the equation:
of the reaction, we use the equation: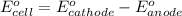
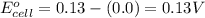
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2[Sn^{2+}]}{[Sn^{4+}]}](/tpl/images/0525/4106/69569.png)
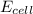 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=1.00M](/tpl/images/0525/4106/641ea.png)
![[Sn^{2+}]=0.350M](/tpl/images/0525/4106/7d5cb.png)
![[Sn^{4+}]=0.020M](/tpl/images/0525/4106/c69f3.png)