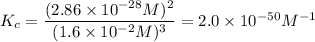Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript...

Chemistry, 26.02.2020 20:55 jessicajamah3289
Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript 3 (g).
At 298 K, the equilibrium concentration of O2 is 1.6 x 10-2 M, and the equilibrium concentration of O3 is 2.86 x 10-28 M. What is the equilibrium constant of the reaction at this temperature?
A) 2.0 x 10^-10
B) 2.0 x 10^10
C) 1.8 x 10^-10
D) 1.8 x 10^10

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Questions

Mathematics, 04.07.2019 15:30

Chemistry, 04.07.2019 15:30

Physics, 04.07.2019 15:30

Health, 04.07.2019 15:30

History, 04.07.2019 15:30


History, 04.07.2019 15:30

Health, 04.07.2019 15:30


Mathematics, 04.07.2019 15:30


Health, 04.07.2019 15:30




Mathematics, 04.07.2019 15:30

Mathematics, 04.07.2019 15:30

Social Studies, 04.07.2019 15:30



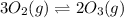
![K_c=\dfrac{[O_3g)]^2}{[O_2(g)]^3}](/tpl/images/0525/4129/a4203.png)