
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the copper half-cell, Cu2+ (aq, 1.00 M) | Cu (s). The hydrogen gas pressure is 1.00 bar. A value of 0.490 V is recorded for E Cell at 298 K. Determine the concentration of H+ and the pH of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2
You know the right answer?
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the co...
Questions



Mathematics, 17.04.2021 21:10

History, 17.04.2021 21:10


Mathematics, 17.04.2021 21:10

Chemistry, 17.04.2021 21:10

History, 17.04.2021 21:10

English, 17.04.2021 21:10

Mathematics, 17.04.2021 21:10

Mathematics, 17.04.2021 21:10


Mathematics, 17.04.2021 21:10


Mathematics, 17.04.2021 21:10

Social Studies, 17.04.2021 21:10


History, 17.04.2021 21:10

Social Studies, 17.04.2021 21:10

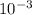 M and the pH = 2.6 of the solution
M and the pH = 2.6 of the solution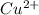 ) is the cathode and hydrogen (
) is the cathode and hydrogen (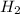 ) is the anode.
) is the anode.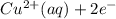 ⇒ Cu(s)
⇒ Cu(s)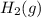 ⇒
⇒ 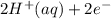
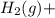
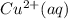 ⇒
⇒ 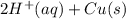
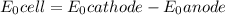
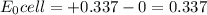
![\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/22043.png)
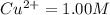 but
but 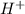 is unknown. we solve this using hernst equation.
is unknown. we solve this using hernst equation.![E = E^{0} -\frac{0.0257}{n}ln\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/8e268.png)
![0.490 = 0.337 -\frac{0.0257}{2}ln\frac{[H^{+}]^{2} }{[1][1]}](/tpl/images/0525/6315/43505.png)
![ln{[H^{+}]^{2} } = -11.9](/tpl/images/0525/6315/8c81e.png)
![2ln{[H^{+}] } = -11.9](/tpl/images/0525/6315/31a40.png)
![ln{[H^{+}] } = -5.95](/tpl/images/0525/6315/e2ee5.png)
![[H^{+}] = 3* 10^{-3} M](/tpl/images/0525/6315/d9bb9.png)


