Chemistry, 26.02.2020 23:22 pennygillbert
Calculate the wavelength of the photon emitted when an electron makes a transition from n=6 to n=3. You can make use of the following constants: h=6.626×10−34 J⋅s c=2.998×108 m/s 1 m=109 nm

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Calculate the wavelength of the photon emitted when an electron makes a transition from n=6 to n=3....
Questions


Biology, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


Spanish, 22.10.2020 18:01


Biology, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


World Languages, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

History, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

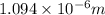
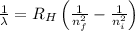
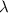 = Wavelength of radiation
= Wavelength of radiation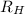 = Rydberg's Constant =
= Rydberg's Constant = 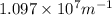
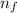 = Final energy level = 3
= Final energy level = 3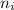 = Initial energy level = 6
= Initial energy level = 6