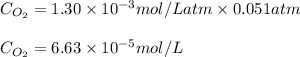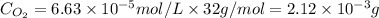Chemistry, 26.02.2020 23:27 josephrosanne18
The partial pressure of in your lungs varies from 25 mm Hg to 40 mm Hg. What mass of can dissolve in 1.0 L of water at 25 °C if the partial pressure of is 39 mm Hg?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
The partial pressure of in your lungs varies from 25 mm Hg to 40 mm Hg. What mass of can dissolve in...
Questions

History, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30




Physics, 28.08.2019 15:30


Health, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30

Chemistry, 28.08.2019 15:30

Chemistry, 28.08.2019 15:30



Mathematics, 28.08.2019 15:30



Business, 28.08.2019 15:30

Social Studies, 28.08.2019 15:30

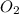 in your lungs varies from 25 mm Hg to 40 mm Hg. What mass of
in your lungs varies from 25 mm Hg to 40 mm Hg. What mass of 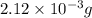
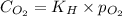
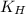 = Henry's constant =
= Henry's constant = 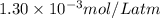
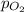 = partial pressure = 39 mm Hg = 0.051 atm (760mmHg=1atm)
= partial pressure = 39 mm Hg = 0.051 atm (760mmHg=1atm)