(View table above)
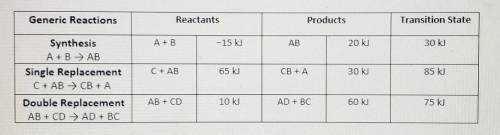
To assist you, use the enthalpy values in the data chart for each gene...

(View table above)
To assist you, use the enthalpy values in the data chart for each generic reaction provided. Be sure to following the summary of steps below.
• Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensure your energy intervals are appropriate for the data
• Plot the enthalpy values of the reactants, products, and transition state using three horizontal dotted lines across the graph for each
• Draw the energy curve from the reactants line to the transition state and curve the line back down to the energy of the products. Label the reactants, products, and transition state.
• Illustrate double-headed arrows to represent both the total change in enthalpy (ΔH) and the activation energy (Ea).
• Calculate the total change in enthalpy and the activation energy using the energy values provided for each reaction. Record those values below the graph.
• Make sure correct units are included.
Conclusion Statement
Write a two to four sentence conclusion statement explaining how the potential energy diagram is used to identify if the reaction is endothermic or exothermic, if heat was released or absorbed, and why the sign of enthalpy change was positive of negative. There should be a conclusion statement for each graph.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Questions

Mathematics, 28.05.2021 05:10


Arts, 28.05.2021 05:10


Mathematics, 28.05.2021 05:10

Chemistry, 28.05.2021 05:10


Mathematics, 28.05.2021 05:10

Physics, 28.05.2021 05:10

Mathematics, 28.05.2021 05:20



English, 28.05.2021 05:20

Chemistry, 28.05.2021 05:20



Mathematics, 28.05.2021 05:20

Mathematics, 28.05.2021 05:20

Social Studies, 28.05.2021 05:20




