
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented in the equation below. Ca(NO3)2(s) + 2NH4Cl(s) → 2N2O(g) + CaCl2(s) + 4H2O(g) What is the maximum volume of N2O at STP that could be produced using a 5.20-mol sample of each reactant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented...
Questions




Health, 28.07.2019 11:00




Social Studies, 28.07.2019 11:00






Mathematics, 28.07.2019 11:00


Physics, 28.07.2019 11:00



Mathematics, 28.07.2019 11:00

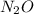 at STP produced is, 116.48 L
at STP produced is, 116.48 L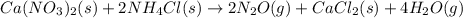
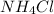 react with 1 mole of
react with 1 mole of 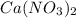
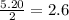 moles of
moles of 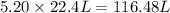 volume of gas
volume of gas 

