Chemistry, 27.02.2020 00:31 dakotakeating4513
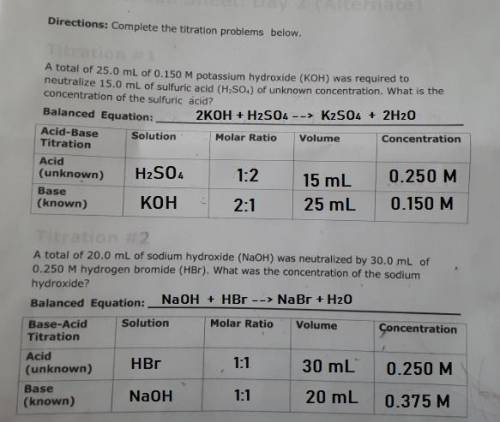
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0 mL of sulfuric acid (H2SO4) of unknown concentration. What is the concentration of the sulfuric acid?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0...
Questions




Mathematics, 06.07.2021 20:40

Social Studies, 06.07.2021 20:40


Mathematics, 06.07.2021 20:40

Social Studies, 06.07.2021 20:40


Mathematics, 06.07.2021 20:40

Mathematics, 06.07.2021 20:40


Mathematics, 06.07.2021 20:40