
Chemistry, 27.02.2020 00:31 paytonrules3634
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. What is the electrode potential, E, of the half reaction if the concentration/activity of all substances is 1.00 M?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. Wha...
Questions


Health, 11.10.2019 08:31

History, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31


Biology, 11.10.2019 08:31

Social Studies, 11.10.2019 08:31

Physics, 11.10.2019 08:31

History, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31




Mathematics, 11.10.2019 08:31


Health, 11.10.2019 08:31




Biology, 11.10.2019 08:31

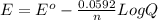
![E = E^o - \frac{0.0592}{n}LogQ\\\\E = 1.13 - \frac{0.0592}{4}Log[1]\\\\E =1.13 -0\\\\E =+1.13 V](/tpl/images/0525/9156/3694d.png)


