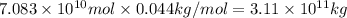Although coal is a complex mixture of substances, its elemental composition can be approximated by the formula . Using this formula, predict the amount of CO₂ released from the combustion of 1.00 × 10⁶ metric tons of coal (about the annual average for a coal-fired power plant). 1 metric ton = 1 ×10³ kg.
a. 3.66 × 10⁹ kg
b. 3.11 × 10⁹ kg
c. 8.50 × 10⁸ kg
d. 3.11 × 10⁵ kg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Although coal is a complex mixture of substances, its elemental composition can be approximated by t...
Questions



Physics, 19.08.2019 08:00

Social Studies, 19.08.2019 08:00

Physics, 19.08.2019 08:00

Mathematics, 19.08.2019 08:00



Social Studies, 19.08.2019 08:00




Mathematics, 19.08.2019 08:00



Mathematics, 19.08.2019 08:00


Mathematics, 19.08.2019 08:00


Mathematics, 19.08.2019 08:00

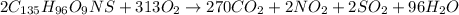
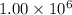 metric ton = 1.00\times 10^6\times 10^3 kg=10^9 kg[/tex]
metric ton = 1.00\times 10^6\times 10^3 kg=10^9 kg[/tex]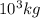
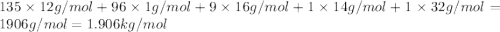
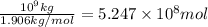
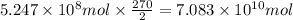 of carbon dioxide.
of carbon dioxide.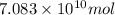 of carbon dioxide:
of carbon dioxide: