
Chemistry, 27.02.2020 01:24 realpcy7515
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3AlCl3), sugar (C6H12O6C6H12O6), benzoic acid (C6H5COOHC6H5COOH), and sodium bromide (NaBrNaBr). Assuming that conductivity is correlated to the number of ions in solution, rank the four substances based on how well a 0.20 MM solution in water will conduct electricity.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3Al...
Questions


Mathematics, 11.11.2019 20:31

Biology, 11.11.2019 20:31


Mathematics, 11.11.2019 20:31

History, 11.11.2019 20:31


Mathematics, 11.11.2019 20:31

Mathematics, 11.11.2019 20:31

Mathematics, 11.11.2019 20:31

Mathematics, 11.11.2019 20:31


English, 11.11.2019 20:31


Biology, 11.11.2019 20:31


Mathematics, 11.11.2019 20:31



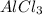 > NaBr > Benzoic acid > sugar
> NaBr > Benzoic acid > sugar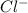 ions and thus, produces 4 ions.
ions and thus, produces 4 ions.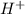 and
and 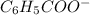 .
.

