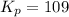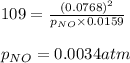The following reaction has Kp = 109 at 25°C. 2 NO(g) + Br2(g) equilibrium reaction arrow 2 NOBr(g) If the equilibrium partial pressure of Br2 is 0.0159 atm and the equilibrium partial pressure of NOBr is 0.0768 atm, calculate the partial pressure of NO at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2


Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
The following reaction has Kp = 109 at 25°C. 2 NO(g) + Br2(g) equilibrium reaction arrow 2 NOBr(g) I...
Questions

Social Studies, 26.10.2021 14:00


English, 26.10.2021 14:00

Chemistry, 26.10.2021 14:00

World Languages, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00


English, 26.10.2021 14:00




English, 26.10.2021 14:00




Mathematics, 26.10.2021 14:00


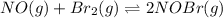
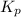 for above equation follows:
for above equation follows: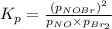
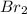 = 0.0159 atm
= 0.0159 atm