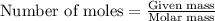Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 Pb(ClO3)2 with aqueous NaI . NaI. Include phases. chemical equation: What mass of precipitate will form if 1.50 L 1.50 L of highly concentrated Pb ( ClO 3 ) 2 Pb(ClO3)2 is mixed with 0.400 L 0.130 M NaI 0.400 L 0.130 M NaI ? Assume the reaction goes to completion

Answers: 2


Another question on Chemistry




Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 Pb(ClO3)2 with aqueous NaI ....
Questions

Advanced Placement (AP), 30.01.2020 14:51









Spanish, 30.01.2020 14:51



Mathematics, 30.01.2020 14:51

Biology, 30.01.2020 14:51


History, 30.01.2020 14:51



Health, 30.01.2020 14:51

Biology, 30.01.2020 14:51

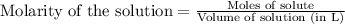
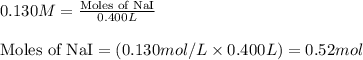
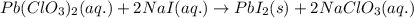
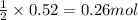 of lead (II) iodide
of lead (II) iodide