
Chemistry, 27.02.2020 02:02 TombRaider167
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid serious problems. One such breathing mixture contains helium, oxygen, and carbon dioxide. Determine the partial pressure of oxygen when the total pressure in the tank is 201.4 kPa if PHe = 125.4 kPa and PCO2= 18.2 kPa? Must show all work that leads to answer for credit

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid s...
Questions

History, 17.11.2020 03:40

History, 17.11.2020 03:40

Mathematics, 17.11.2020 03:40

Health, 17.11.2020 03:40

Mathematics, 17.11.2020 03:40

History, 17.11.2020 03:40



Mathematics, 17.11.2020 03:40


Chemistry, 17.11.2020 03:40


Chemistry, 17.11.2020 03:40



Business, 17.11.2020 03:40

Mathematics, 17.11.2020 03:40

Mathematics, 17.11.2020 03:40


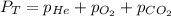
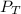 = 201.4 kPa
= 201.4 kPa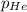 = 125.4 kPa
= 125.4 kPa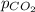 = 18.2 kPa
= 18.2 kPa![201.4=125.4+p_{O_2}+18.2\\\\p_{O_2}=201.4-[125.4+18.2]=57.8kPa](/tpl/images/0526/1098/fa869.png)


