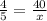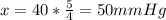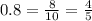Chemistry, 27.02.2020 03:16 xlebrny7831
A man with normal lungs and an arterial PO2 of 40 mmHg takes an overdose of barbiturates that halves his alveolar ventilation without changing his metabolism. If his respiratory exchange ratio is 0.8, how much does his inspired oxygen concentration (%) have to be increased to return his alveolar PO2 to the original level?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
A man with normal lungs and an arterial PO2 of 40 mmHg takes an overdose of barbiturates that halves...
Questions

Geography, 25.09.2019 09:50

Health, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50


Social Studies, 25.09.2019 09:50




Spanish, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Social Studies, 25.09.2019 09:50


Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50

Mathematics, 25.09.2019 09:50



Biology, 25.09.2019 09:50

English, 25.09.2019 09:50