Chemistry, 27.02.2020 03:31 kaitlyn114433
Each isotope has a unique half-life. The half-life of an isotope is the time taken for half of the starting quantity to decay (with a ratio of 1:1). After two half-lives, there will be one-fourth of the original parent sample and three-quarters would have decayed to the daughter product (with a ratio of 1:3). After three half-lives, the ratio becomes 1:7, and so forth.
A graph showing months on the x-axis and the amount of parent/daughter on the y-axis. The graph uses four pie charts to demonstrate how parent elements and daughter elements change with each half life for a sample in increments of 4 months.
The graph, for instance, shows that assuming the half-life of a sample is 4 months, then in 4 months, there will be 0.5 gram of the parent element and 0.5 gram of the daughter element will be produced. In month 8 (which is two-half-lives), there will be only 0.25 gram of parent element left and 0.75 gram of daughter element; that is, one-fourth of the parent sample (in red) is left, and in month 12, there is only one-eighth of the parent element. You attend a geology lab where you are asked to estimate the age of a fossil. The ratio of parent to daughter elements in the fossil sample is 1:7. You know that fossils are the remains of living organisms, which have some amount of C-14 isotope. The C-14 isotope, which has a half-life of 5730 years, begins to decay as the organism dies. What would be your estimation of the fossil's age?a. 2865
b. 17,190
c. 5730
d. 11,460
e. 40,110
f. 22,920

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
Each isotope has a unique half-life. The half-life of an isotope is the time taken for half of the s...
Questions

Advanced Placement (AP), 14.12.2019 19:31




Mathematics, 14.12.2019 19:31





Chemistry, 14.12.2019 19:31


Mathematics, 14.12.2019 19:31

History, 14.12.2019 19:31




Mathematics, 14.12.2019 19:31

Mathematics, 14.12.2019 19:31

Mathematics, 14.12.2019 19:31

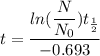
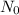 =1
=1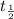 =5730 years
=5730 years