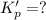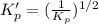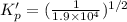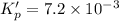Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
For the following reaction, Kp = 1.9 ✕ 104 at 1722 K. H2(g) + Br2(g) equilibrium reaction arrow 2 HB...
Questions








Mathematics, 13.11.2020 18:10



Mathematics, 13.11.2020 18:10



English, 13.11.2020 18:10

Mathematics, 13.11.2020 18:10


Mathematics, 13.11.2020 18:10

Mathematics, 13.11.2020 18:10


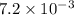
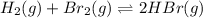 ;
; 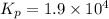
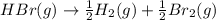 ;
;