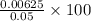Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0390 M Ag + ( a...
Questions

Mathematics, 02.03.2021 19:40



Mathematics, 02.03.2021 19:40

Chemistry, 02.03.2021 19:40

Mathematics, 02.03.2021 19:40

English, 02.03.2021 19:40


Mathematics, 02.03.2021 19:40

Geography, 02.03.2021 19:40

SAT, 02.03.2021 19:40



Mathematics, 02.03.2021 19:40

Biology, 02.03.2021 19:40



Mathematics, 02.03.2021 19:40


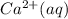 and 0.0390 M
and 0.0390 M 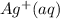 . What will be the concentration of
. What will be the concentration of 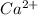 (aq) when
(aq) when 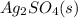 begins to precipitate? What percentage of the
begins to precipitate? What percentage of the 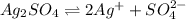
![[Ag^{+}]](/tpl/images/0526/4790/52db7.png) = 0.0390 M
= 0.0390 M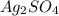 precipitates then expression for
precipitates then expression for 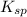 will be as follows.
will be as follows.![K_{sp} = [Ag^{+}]^{2}[SO^{2-}_{4}]](/tpl/images/0526/4790/4148c.png)
![1.20 \times 10^{-5} = (0.0390)^{2} \times [SO^{2-}_{4}]](/tpl/images/0526/4790/e9f5a.png)
![[SO^{2-}_{4}]](/tpl/images/0526/4790/a4a68.png) = 0.00788 M
= 0.00788 M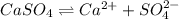
![K_{sp} = [Ca^{2+}][SO^{2-}_{4}]](/tpl/images/0526/4790/0d847.png)
![4.93 \times 10^{-5} = [Ca^{2+}] \times 0.00788](/tpl/images/0526/4790/02a50.png)
![[Ca^{2+}]](/tpl/images/0526/4790/17576.png) = 0.00625 M
= 0.00625 M