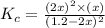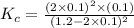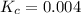Chemistry, 27.02.2020 05:23 jamccoy3335
At a certain temperature, 0.800 mol SO 3 is placed in a 1.50 L container. 2 SO 3 ( g ) − ⇀ ↽ − 2 SO 2 ( g ) + O 2 ( g ) At equilibrium, 0.150 mol O 2 is present. Calculate K c .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2


Chemistry, 23.06.2019 15:10
Certain types of organisms such as fireflies and anglerfish can produce light through chemical reactions in a process called bioluminescence. what kind of chemical reactions occur during bioluminescence? o a) exothermic ob) endothermic oc) recomposition od) decomposition
Answers: 2
You know the right answer?
At a certain temperature, 0.800 mol SO 3 is placed in a 1.50 L container. 2 SO 3 ( g ) − ⇀ ↽ − 2 SO...
Questions

Mathematics, 17.06.2020 03:57




Arts, 17.06.2020 03:57

English, 17.06.2020 03:57

Mathematics, 17.06.2020 03:57

History, 17.06.2020 03:57


Mathematics, 17.06.2020 03:57

Advanced Placement (AP), 17.06.2020 03:57


English, 17.06.2020 03:57




Mathematics, 17.06.2020 03:57



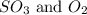
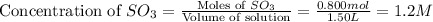
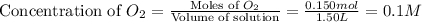
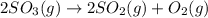
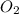 at equilibrium = x = 0.1 M
at equilibrium = x = 0.1 M![K_c=\frac{[SO_2]^2[O_2]}{[SO_3]^2}](/tpl/images/0526/5076/ea3f9.png)