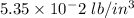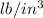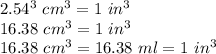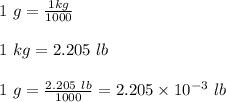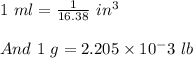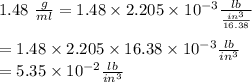Chemistry, 27.02.2020 05:47 faithyholcomb
11. The density of liquid chloroform is 1.48 g/mL. What is its density in units of lb/in?
(2.54 cm = 1 in., 2.205 lb = 1 kg)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
11. The density of liquid chloroform is 1.48 g/mL. What is its density in units of lb/in?
Questions

Mathematics, 20.09.2020 02:01


English, 20.09.2020 02:01

Physics, 20.09.2020 02:01



English, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Geography, 20.09.2020 02:01


Geography, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01


German, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

English, 20.09.2020 02:01