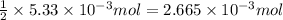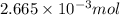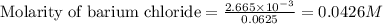A 20.00 mL Ba ( OH ) 2 solution of unknown concentration was neutralized by the addition of 42.50 mL of a 0.1255 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba ( OH ) 2 in aqueous solution. Include physical states. molecular equation: Ba^{2+}(aq) +2OH^{-}(aq) +H^{+}(aq) +Cl^{-}(aq)<=>H_{2}O(l) +Ba^{2+}(aq) +Cl^{-}(aq) Ba 2 + ( aq ) + 2 OH − ( aq ) + H + ( aq ) + Cl − ( aq ) − ⇀ ↽ − H 2 O ( l ) + Ba 2 + ( aq ) + Cl − ( aq ) Calculate the concentration of Ba ( OH ) 2 in the original 20.00 mL solution. [ Ba ( OH ) 2 ] = M Calculate the concentrations of Ba 2 + and Cl − in solution following the neutralization reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
A 20.00 mL Ba ( OH ) 2 solution of unknown concentration was neutralized by the addition of 42.50 mL...
Questions

Mathematics, 20.09.2019 05:10

Computers and Technology, 20.09.2019 05:10

Social Studies, 20.09.2019 05:10

Chemistry, 20.09.2019 05:10






Mathematics, 20.09.2019 05:10


Chemistry, 20.09.2019 05:10







Computers and Technology, 20.09.2019 05:10

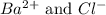 ions in the solution are 0.0426 M and 0.0852 M respectively
ions in the solution are 0.0426 M and 0.0852 M respectively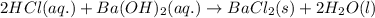
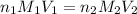
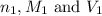 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl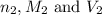 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 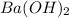
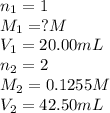
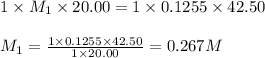
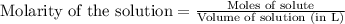 .....(1)
.....(1)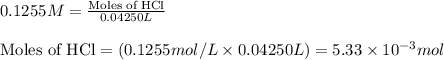
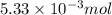 of HCl will produce =
of HCl will produce =