
Chemistry, 27.02.2020 05:57 davidsouth444
Chlorine gas reacts with phosphorus to produce phosphorus pentachloride. How many grams of PCl5 are produced from 3.5 g of Cl2 and excess P? Cl2(g) + P(s) --> PCl5(s) ( unbalanced)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Chlorine gas reacts with phosphorus to produce phosphorus pentachloride. How many grams of PCl5 are...
Questions

Mathematics, 02.11.2020 04:20



History, 02.11.2020 04:20

Geography, 02.11.2020 04:20

Mathematics, 02.11.2020 04:20

Mathematics, 02.11.2020 04:20


Mathematics, 02.11.2020 04:20

Mathematics, 02.11.2020 04:20

Business, 02.11.2020 04:20


Chemistry, 02.11.2020 04:20



Mathematics, 02.11.2020 04:20


Mathematics, 02.11.2020 04:20


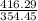 gram of PCl₅
gram of PCl₅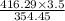 gram of PCl₅
gram of PCl₅

