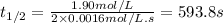The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. It is the time during which the amount of reactant or its concentration decreases to one-half of its initial value.
Determine the half-life for the reaction in Part B using the integrated rate law, given that the initial concentration is 1.90mol?L?1 and the rate constant is 0.0016mol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. I...
Questions

Chemistry, 15.01.2021 17:00



Mathematics, 15.01.2021 17:00

Mathematics, 15.01.2021 17:00

English, 15.01.2021 17:00


Mathematics, 15.01.2021 17:00



Mathematics, 15.01.2021 17:00

Mathematics, 15.01.2021 17:00

Mathematics, 15.01.2021 17:00

Biology, 15.01.2021 17:00


Social Studies, 15.01.2021 17:00


Arts, 15.01.2021 17:00


Mathematics, 15.01.2021 17:00


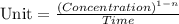

![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0526/7626/b5b11.png)
![[A_o]](/tpl/images/0526/7626/dc622.png) = initial concentration = 1.90 mol/L
= initial concentration = 1.90 mol/L