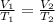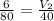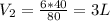Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
1. A given mass of air has a volume of 6.00 L at 80.0°C. At constant pressure, the temperature is
Questions



Mathematics, 21.05.2021 21:20


Mathematics, 21.05.2021 21:20

Physics, 21.05.2021 21:20


Mathematics, 21.05.2021 21:20



Social Studies, 21.05.2021 21:20

Mathematics, 21.05.2021 21:20

History, 21.05.2021 21:20



Mathematics, 21.05.2021 21:20

Mathematics, 21.05.2021 21:20


Mathematics, 21.05.2021 21:20