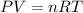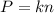Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g of Ne, and the other flask contains 10.0 g of He. Is each of the following statements true or false? Explain.1) True. Because the gases have the same mass, they exert the same pressures.2) True. Both the volumes and the temperatures of the gases are the same, which means that their pressures must be equal.3) False. There are different numbers of moles in the flasks, meaning that the pressures are different.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. One flask contains 1...
Questions

Mathematics, 04.12.2020 18:40




Biology, 04.12.2020 18:40


Health, 04.12.2020 18:40


Mathematics, 04.12.2020 18:40

Biology, 04.12.2020 18:40

English, 04.12.2020 18:40







Arts, 04.12.2020 18:40